 Beretta
Berettaможно решение хотя бы одного из 3 и 4
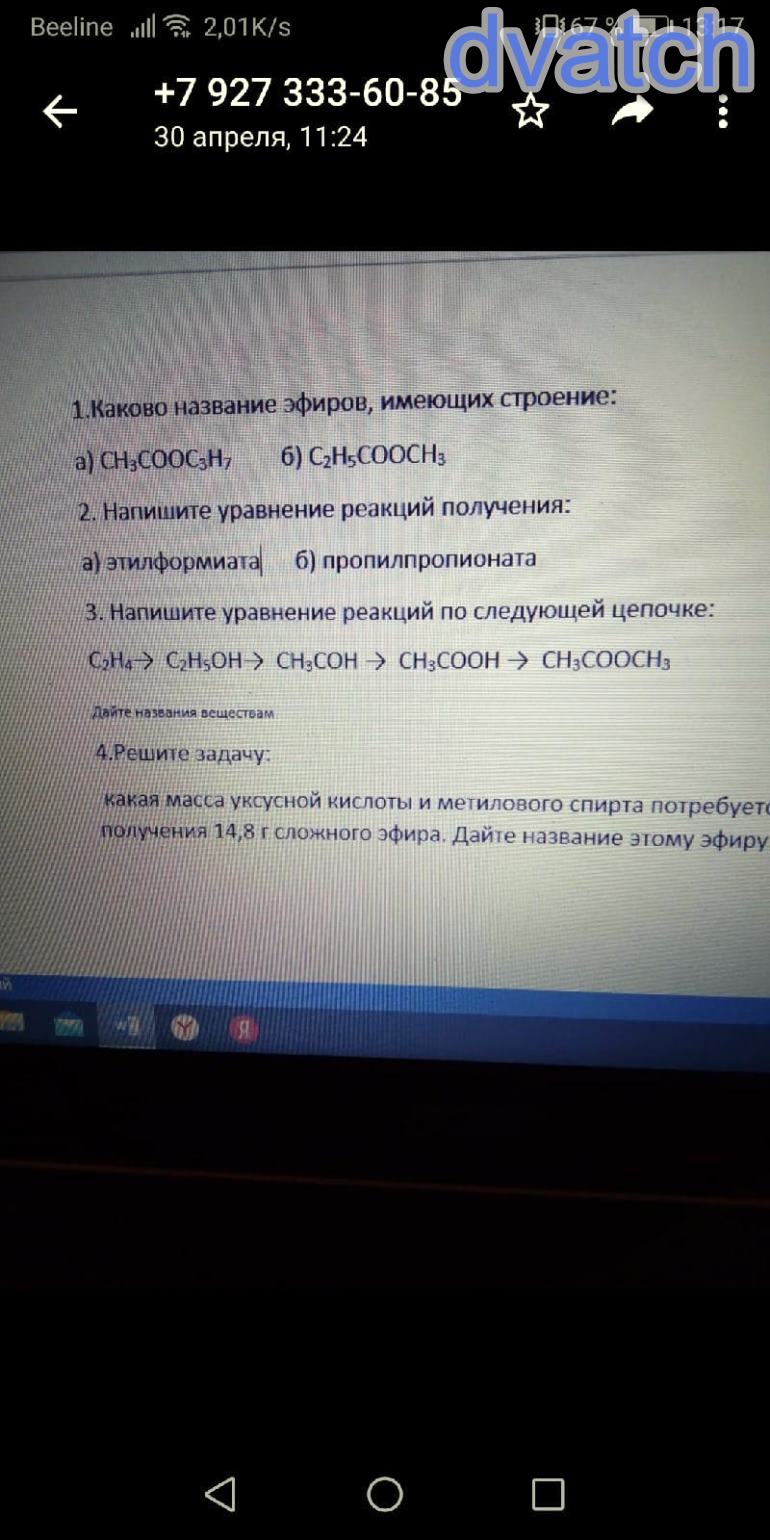
ответы: 1
Зарегистрируйтесь, чтобы добавить ответ
Ответ:
#3
ch2=ch2+h2o->{h+} ch3-ch2-oh
ch3-ch2-oh+cuo->{t°} cu+h2o+ch3-coh (альдегид)
ch3-coh+ag2o->{t°, p-p nh3} ch3-cooh+2ag
ch3-cooh+ch3-oh->{h2so4, t°} ch3-cooch3+h2o
#4
ch3-cooh+ch3-oh->{h2so4, t°} ch3-cooch3+h2o
n(ch3-cooch3)=m(ch3-cooch3)/M(ch3-cooch3)=14. 8г/74г/моль=0. 2 моль
из ур-ния реакции:
n(ch3cooh)=n(ch3-cooch3)=0. 2 моль
m(ch3cooh)=n(ch3cooh)*M(ch3cooh)=0. 2моль*60г/моль=12г
330
 Hermansso
HermanssoЧтобы ответить необходимо зарегистрироваться.